Herbal Medicine vs. Antibiotics: Why Science Is Circling Back
- Petal & Root

- Oct 8
- 5 min read
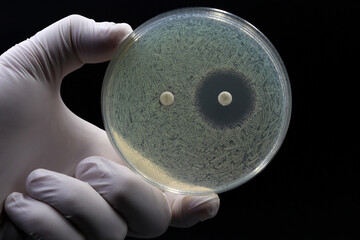
As an herbalist, I understand the deep respect many hold for pharmaceutical antibiotics which in many cases can be lifesaving tools. But I also see a growing danger: antibiotic resistance is eroding their power. So, the controversial but necessary question arises: can herbal medicine offer a credible alternative or complement in a post-antibiotic age?
In this post, I’ll explore how herbs might support or even enhance antibiotic therapy, examine the limits and risks of herbal antimicrobials, and push into hard questions few ask: Are we overusing herbs? Could microbes “learn” herbs too? When does a serious infection demand modern medicine?
The Crisis: Antibiotic Resistance
Antibiotic resistance is one of the top public health threats of our time. Overuse and misuse (e.g. in agriculture, low dose exposure, incomplete courses) has driven pathogens to evolve defenses. Some “superbugs” now resist our strongest antibiotics.
I am not advocating for the abandonment of antibiotics, but it is vital we seek new strategies, and enhance their stewardship.
How Herbs Can Help (Backed by Science)

1. Intrinsic antimicrobial activity
Many plants produce compounds toxic to microbes (alkaloids, phenolics, essential oils).
For instance:
• A 2009 paper documented that five herbal extracts inhibited multi-drug resistant bacterial strains in vitro. MDPI
• Ruta graveolens extract demonstrated antimicrobial and biofilminhibiting effects against MRSA, and downregulated genetic resistance markers (e.g. mecA) in lab tests. NCBI
• A recent review “Herbal medicine: the magic way crouching microbial resistance” highlights nine herbs that may act synergistically with antibiotics to reverse resistance mechanisms. Taylor & Francis
• A 2025 article in Frontiers in Cellular and Infection Microbiology describes how traditional Chinese medicine compounds may inhibit bacterial quorum sensing (the communication system enabling bacterial cooperation and resistance) to reverse antibiotic resistance. Frontiers
These lab and mechanistic studies are intriguing, but they are not the same as evidence in humans with real infections.
2. Synergy with antibiotics (resistance modifying effect)
Some herbal compounds don’t kill pathogens outright but enhance antibiotic efficacy or reverse resistance:
• A 2022 review found that herbal products or active constituents (e.g. berberine, Piper betle extract) could reduce the minimum inhibitory concentration (MIC) of antibiotics against resistant strains, acting as adjuvants. PubMed
• Turnera ulmifolia extract was shown to potentiate antibiotic activity against MRSA in lab settings. BioMed Central
• A broader review on plant antimicrobials emphasizes that many phytochemicals act both as antimicrobials and antibiotic resistance modifiers. BioMed Central
So, herbs might help “rescue” antibiotics in some settings, but not replace them entirely.
3. Multiple modes of action prevent resistance
Antibiotics often have a single target (e.g. cell wall, protein synthesis). In contrast, many herbs act via multi-target pathways (membrane disruption, enzyme inhibition, biofilm breakdown, efflux pump inhibition). This multi-pronged assault may slow or prevent the development of resistance. BioMed Central, Taylor & Francis
As one review notes: "When combined with antibiotics, natural compounds can reduce MICs and suppress development of resistance." BioMed Central
What We Don’t Know (And Should Admit)

• Lack of human trials: Almost all strong data are in vitro or animal models. Very few well controlled human trials exist to prove herbal antimicrobials can replace or significantly reduce antibiotic dosing in serious infections.
• Dosing, safety, and standardization: Herbal extracts vary widely in strength, purity, and constituents. Without standardization, reproducibility is difficult.
• Toxicity and interaction risk: Some herbs can harm the liver, kidneys, or interact negatively with medications. Always assume herbs have biochemical potency.
• Resistance to herbs: It’s less well studied, but theoretically bacteria may adapt over time. Overuse of any antimicrobial, synthetic or botanical, could push microbial evolution.
• Infection severity boundary: Herbs may help mild to moderate infections or serve prophylactically, but for deep-seated, rapidly progressive infections (e.g. bloodstream infections, pneumonia, sepsis), antibiotics (and medical care) remain essential.
Hard Questions Most Don’t Ask But Should
Q: If herbs help, why aren’t clinicians widely using them already?
Because the regulatory and evidentiary bar is high. Medicine requires randomized controlled trials, safety profiling, and dose standardization. Herbal medicine struggles with that because of complexity, funding constraints, and variable botanical sources. But the tide is slowly shifting as integrative medicine gains traction.
Q: Could overuse of herbal antimicrobials drive herbal resistance?
It’s certainly possible; any antimicrobial pressure selects survivors. This is why rotation, prudent use, and combination therapy are hallmarks of good stewardship, whether with drugs or botanicals.
Q: Which infections are best suited for herbal and antibiotic synergy?
Likely surface infections, recurrent urinary tract infections, mild skin and soft tissue infections, or prophylaxis in vulnerable populations. Probably not advanced hospital-acquired sepsis on its own.
Q: How do I, as a patient or consumer, responsibly “use herbs” without creating problems?
• Start mild and rotate herbs rather than rely on one formula long term.
• Combine with proven therapies (e.g. antibiotics where needed) rather than “either/or.”
• Work with professionals who understand both herbal and conventional medicine.
• Monitor clinical signs, lab markers, and adjust or stop if there is no progress.
My Approach as a Responsible Herbalist

When I design herbal protocols in settings where infection is a concern, I follow these principles:
1. Use synergy over substitution: I rarely advocate replacing antibiotics outright unless in extremely low-risk cases; rather, I use herbs to support, modulate, and reduce antibiotic burden where safely possible.
2. Use complex herbal formulas (not single isolated compounds) so resistance pressure is diffused across multiple modes of action.
3. Cycle and rotate herbs to minimize selective pressure on microbes.
4. Monitor carefully: track symptoms, microbial cultures when possible, biomarkers (e.g. CRP, WBC), and end points (healing, recurrence).
5. Respect escalation thresholds: if an infection worsens or fails to respond, I do not delay recommending or referring for antibiotics or medical intervention.
Case Illustration (Hypothetical)
Imagine a client with recurrent urinary tract infections (UTIs) of mild to moderate severity, previously treated frequently with antibiotics. I might propose:
• a supportive herbal formula containing known urinary antimicrobials (e.g. bearberry, uva ursi, cleavers, horsetail)
• biofilm disruptors (e.g. enzymes, D-mannose)
• nutritional and immune support (vitamin D, probiotics, hydration)
• careful monitoring and urine testing
If symptoms resolve quickly, no further antibiotics may be needed. But if fever, worsening signs, or blood infection appear, I would absolutely refer out for medical care.
The Bridge Ahead: Integrative Stewardship
I believe the future lies in integrative antimicrobial stewardship: combining the precision of pharmaceuticals with the resilience of botanicals. As more high-quality research emerges, we may see protocols where antibiotic doses are scaled down, side effects are reduced, and resistance is slowed all with the support of wise herbaceous allies.
Until then, we must walk humbly. Herbs aren’t magic bullets; they are potent biochemical tools that deserve respect, not hype. Used wisely, they can help preserve the power of antibiotics, extend their lifespan, and reimagine a future where we don’t pit modern medicine vs plants but instead build bridges between them.
Stay Radiant,









